Effects of Protein Conformation, Apparent Solubility, and Protein-Protein Interactions on the Rates and Mechanisms of Aggregation for an IgG1Monoclonal Antibody
Abstract
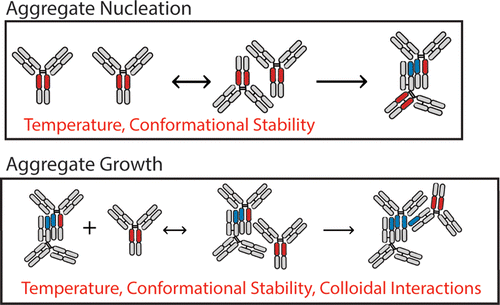
Non-native protein aggregation is a key degradation pathway of immunoglobulins. In this work, the aggregation kinetics of an immunoglobulin gamma-1 monoclonal antibody (IgG1 mAb) in different solution environments was monitored over a range of incubation temperatures for up to seven months using size exclusion chromatography. Histidine and citrate buffers with/without sodium chloride were employed to modulate the mAb’s conformational stability, solubility (in the presence of polyethylene glycol, PEG), and protein-protein interactions as measured by differential scanning calorimetry, PEG precipitation, and static light scattering, respectively. The effect of these parameters on the mechanism(s) of mAb aggregation during storage at different temperatures was determined using kinetic models, which were used to fit aggregation data to determine rate constants for aggregate nucleation and growth processes. This approach was used to investigate the effects of colloidal protein-protein interactions and solubility values (in PEG solutions) on the mechanisms and rates of IgG1 mAb aggregation as a function of temperature-induced structural perturbations. Aggregate nucleation and growth pathways for this IgG1 mAb were sensitive to temperature and overall conformational stability. Aggregate growth, on the other hand, was also sensitive to conditions affecting the solubility of the mAb, particularly at elevated temperatures.