The N-terminus of IpaB provides a potential anchor to the Shigella type III secretion system tip complex protein IpaD
Abstract
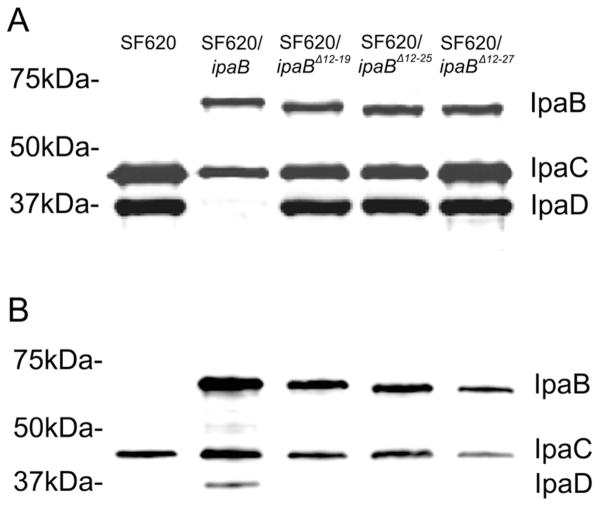
The type III secretion system (T3SS) is an essential virulence factor for Shigella flexneri, providing a conduit through which host-altering effectors are injected directly into a host cell to promote uptake. The type III secretion apparatus (T3SA) is comprised of a basal body, external needle, and regulatory tip complex. The nascent needle is a polymer of MxiH capped by a pentamer of invasion plasmid antigen D (IpaD). Exposure to bile salts (e.g. deoxycholate) causes a conformational change in IpaD and promotes recruitment of IpaB to the needle tip. It has been proposed that IpaB senses contact with host cell membranes, recruiting IpaC and inducing full secretion of T3SS effectors. While the steps of T3SA maturation and their external triggers have been identified, details of specific protein interactions and mechanisms have remained difficult to study due to the hydrophobic nature of the IpaB and IpaC translocator proteins. Here we explored the ability for a series of soluble N-terminal IpaB peptides to interact with IpaD. We found that DOC is required for the interaction and that a region of IpaB between residues 11–27 is required for maximum binding, which was confirmed in vivo. Furthermore, intramolecular FRET measurements indicated that movement of the IpaD distal domain away from the protein core accompanied the binding of IpaB11-226. Together these new findings provide important new insight into the interactions and potential mechanisms that define the maturation of the Shigella T3SA needle tip complex and provide a foundation for further studies probing T3SS activation.